Mandsaur Institute of Pharmacy
Departmental Activity
World Pharmacist Day (Rally & Nukkad Natak)
Second Winner in NMPB Sponsored National Conference at Amaravati [MH]
Pharmacist day celebration
One day National seminar and poster competition on ‘Popularization of Pharmacy & Health Education’ sponsored by Madhya Pradesh Council for Science and Technology (MPCST)
About Mandsaur Institute of Pharmacy
Mandsaur Institute of Pharmacy (MIP) is nurtured by B. R. Nahata Smriti Sansthan (BRNSS), a philanthropic organization committed to the service of humanity through technological advancement. The institute carries all the necessary approvals of Pharmacy council of India, New Delhi and is affiliated to Mandsaur University from year 2016, before it was affiliated to Rajiv Gandhi Technical University (RGPV), Bhopal.
Faculty of Pharmacy has an aim to provide excellent facilities for education and research. The objective of the institute is to provide solid scientific and technical knowledge required to prepare professionals capable of undertaking with responsibility and professional qualification.
When it comes to research we have been at the forefront ever since we launched the first private player run post graduate program in pharmaceutical sciences in the state with phenomenal outcome in publication, presentations and research projects from budding pharmacists and research scholars. We advocate the skill – based education rather than rote learning or syllabus driven or exam oriented approach. The academic programs have been bench-marked against the best prevailing standards with regular up-gradation. It is our belief that value-based learning leading to total development of personality and expertise of the students and the faculty will be our long lasting contribution to the overall growth of our nation and society.
The Faculty has an advanced infrastructural facility like sophisticated instrumentation lab, Biotechnology and microbiology labs, advanced chemistry facilities lab and other state of art research laboratories.
Pharmacy is a fast growing sector worldwide. Advances in medical and pharmaceutical sciences can be felt in day to day life. Thus there has been a tremendous increase in the job opportunities of pharmacy graduates, post graduates and researchers.
Departmental Gallery

Dean's Message
Welcome to the Faculty of Pharmacy, Mandsaur University. Our campus has grown from its inception to accommodate students in first –class teaching facilities which are amid beautifully kept grounds.
We are fortunate to have a talented, highly committed teaching and support staff here the learning environment for our students is best it can be. Our University provides the fabric with which the tapestry of our student’s experiences unfolds.
It is our belief that value based learning leading to total development of personality and expertise of the students and the faculty, will be our long lasting contribution to the growth of our nation and Society.
We seek to prepare our young students with very best preparation for life after college. We seek to install in our students a passion for leaning that will bring the knowledge and understanding, they will need to make a positive contribution to the communities in which they live and work.
The likelihood of achieving this is strengthened by the fact that we offer an academic program that includes breadth and depth is rigorous, and which can be tailored to individual needs.
In case you are aspirant of making career in pharmacy profession, we are pleased to abreast you of the emergent issues in the profession as well as the endeavors of Faculty of Pharmacy, Mandsaur University in sculpturing the profession and its seekers.
Dr. Naveen Kumar Choudhary
M.Pharm, PhD
Principal, MIP,Mandsaur
Courses
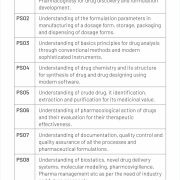
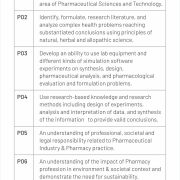
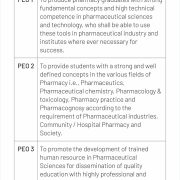
PEO, PSO & PO
Why Study Pharmacy
Duration: 4 years (Semester System)
AFFILIATION
Constituent College of MU
ELIGIBILITY CRITERIA
The candidate shall have passed HSC or 10+2 examination from (PCM/B) stream. For B. Pharm.
MODE OF ADMISSION
For admissions please visit our portal to register/login yourself and proceed ahead with your admission application.
laboratories

Pharmaceutics Lab
All the laboratories having good ventilation and lighting facilities along with continuous water supply. The laboratories are designed efficiently for proper storage of chemicals and glassware. These laboratories are well equipped with the latest instruments, and appliances.

Pharmacology Lab
For the approval of various research projects on experimental animals the department has centralized animal house approved by CPCSEA, New Delhi for conducting experiments on Mice, Rats, Guinea Pigs, and Rabbits. Animal house is maintained with proper housing condition for experimentation.

Pharmaceutical Chemistry Lab
The Pharmaceutical chemistry department is committed to excellence in Pharmaceutical Chemistry through providing a nurturing and conducive environment for teaching and learning in basic and medicinal chemistry.

Pharmacognosy Lab
The subject is mainly concerned with the study of drugs obtained from the natural source, provides information about the principles and procedures used in the identification, authentication, extraction, isolation of active constituents by chromatography techniques, purification and development of herbal formulations and assay methods for the isolated constituents.

Pharmaceutical Analysis Lab
Pharmaceutical Analysis department of the institute will ensure an environment for teamwork and successful organizational practices. The Department is well equipped with modern analytic facilities like U.V, HPTLC, HPLC, FT-IR etc.

Biotechnology and Microbiology Lab
The Biotechnology and Microbiology Laboratory offers state-of-the-art facilities and hands-on activities to help students better understand the promising approach of biotechnology and microbiology and learn its tools and techniques.
R&D Activities
Recruiters










Faculties


Dr. M a Naidu
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Dr. Swapnil Goyal
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Ms.Kajal Mudgal
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mr.Aadil Hussain
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Ms Akansha Porwal
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mrs.Anubha Rajawat
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mr.Chandra Kant Mod
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mr.Javed Mansuri
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Ms.Chandrakala Kiloria
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Ms.Manisha Nath
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mr.Rahul Kaushik
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mrs.Shivani Sharma
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mr.Saurabh Sethiya
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)
Mr.Sunil Thakar
Assistant Professor, Mandsaur Institute of Pharmacy, Mandsaur (M.P)

![Second Winner in NMPB Sponsored National Conference at Amaravati [MH] Second Winner in NMPB Sponsored National Conference at Amaravati [MH]](https://www.meu.edu.in/wp-content/uploads/2022/10/Winner-Certificate-554x674.jpg)